
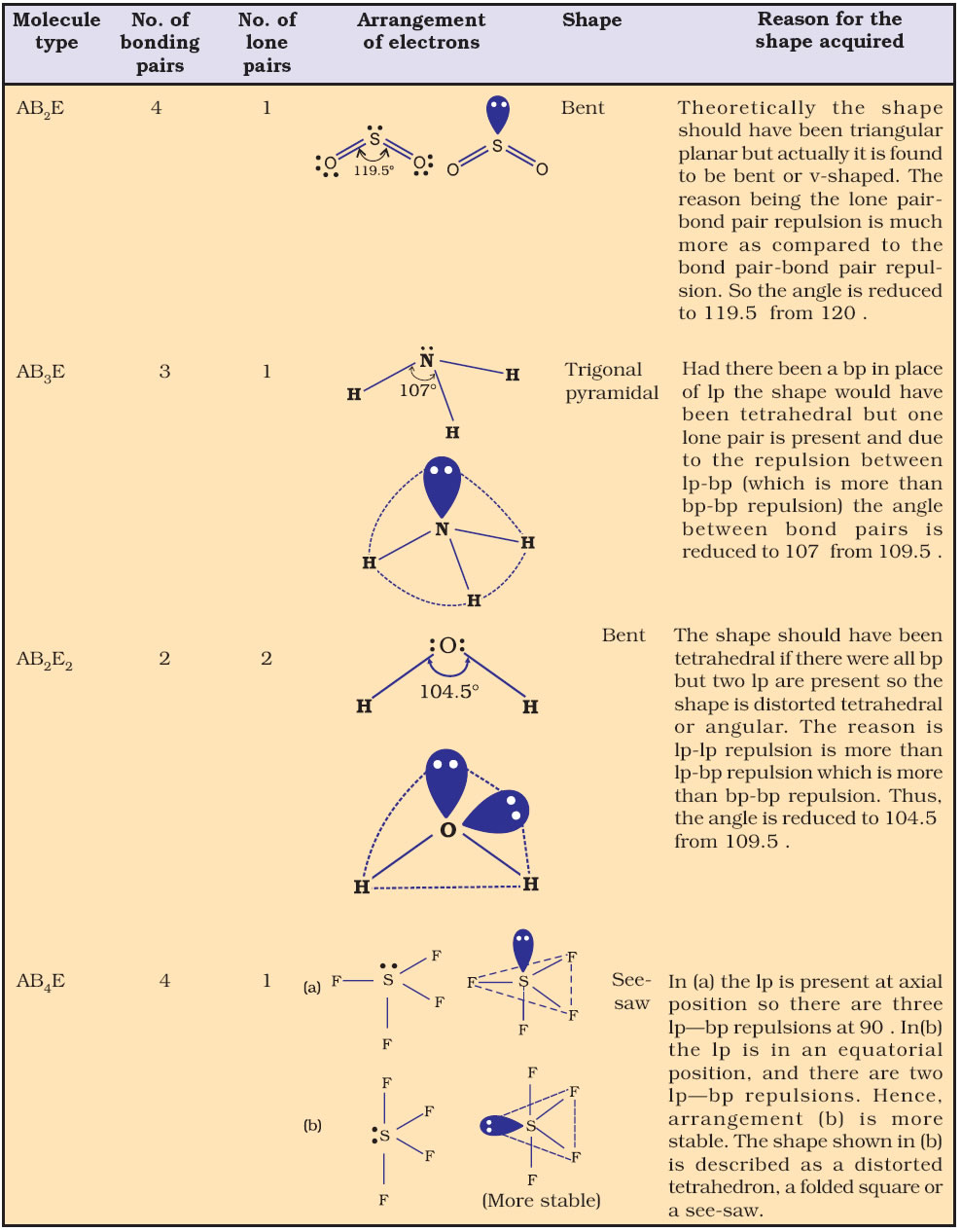
What you are going to focus on to determine the molecular shape of a molecule is the central atom from your electron dot structure. How do you determine the molecular shapes (molecular geometry) from the electron dot structure (Lewis structure)? The theory of electrons repelling each other to create the shape of the molecule is called the VSEPR (Valence Shell Electron Pair Repulsion) model. This is driven by the electrons in the bonds and lone pairs of the central atom repelling each other and trying to maintain the furthest distance apart as possible. Many molecules but not all you create by electron dot structures (Lewis structures) exist not a flat two dimensional molecules like you drew them on a piece of paper but occupy 3 dimensions. T-shaped 90° (87.How do electron dot structures (Lewis structures) relate to the molecular (3 dimensional) shapes? The following chart shows the ideal and observed bond angles for different molecular shapes when lone pairs are introduced into the basic shape. The overall shape of the molecule is bent or angular. The two lone pairs will repel the bond pairs further, thereby reducing the bond angle to 104.5°. Water (H 2O) has two bond pairs and two lone pairs.The resultant molecular shape is trigonal pyramidal. The lone pair will push the bond pairs outwards, reducing the bond angle to 107°. Ammonia (NH 3) consists of three bond pairs and one lone pair.Therefore, its shape is tetrahedral with a standard bond angle of 109.5°. Methane (CH 4) consists of all bond pairs.Let us see how the shape changes from methane to ammonia to water. Four electron pairs (bonding and nonbonding) are required to define a tetrahedral-shaped molecule whose standard bond angle is 109.5°. Let us consider the tetragonal shape to understand this effect.

Therefore, the bond angles of some molecules depend on the number of lone pairs. The lone pairs are located in the atomic orbitals of the central atom and repel the bond pairs, causing a deviation from the abovementioned geometry. Lone pairs of electrons on the central atom affect the molecular shape. īond Angles Effect of Lone Pairs on Bond Angle Bond angles can distinguish these five shapes, as shown in the table below. The VSEPR theory predicts five fundamental shapes of molecules with no lone pairs. The angle between the adjacent bonds defines the bond angle. As a result, the molecule forms a regular geometric shape. According to this theory, the lone pairs in the valence shell of the central atom will rearrange themselves to minimize the repulsion and maximize the distance between them. This theory predicts a molecule’s shape based on the number of bonding and lone pairs. The valence shell electron pair repulsion (VSEPR) theory is used to study bond angles. The bond angle is the angle between any two adjacent bonds and is usually measured in degrees.
A molecule consists of a central atom chemically bonded to several side atoms.

In our article on molecular geometry, we discovered how atoms are arranged in a molecule in a two- or three-dimensional structure.


 0 kommentar(er)
0 kommentar(er)
